News
Experience with long-term antibiosis in late borreliosis and arguments for it - knowledge about biofilms as a possible cause of chronic infections
Presentation at the spring meeting of the German Borreliose Society, Schweinfurt, April 2012
Wolfgang Klemann
No evidence-based studies are available for the treatment of chronic Lyme borreliosis and relevant co-infections; the recommendations of the various specialist societies are based solely on expert opinion. So there are currently no satisfactory standards for the treatment of chronic Lyme disease and relevant co-infections; This applies in particular to patients in whom short-term antibiotic therapy did not bring any real recovery.
17 years ago, when I was confronted with a severe case of neuroborreliosis (plexus neuritis) for the first time, the recommendations for treatment were already controversial. Joseph Burrascano ( 1 ) was one of the few authors who recommended long-term antibiotic therapy at the time.
To stay with the severe case of neuroborreliosis mentioned: On the occasion of the stationing that became necessary at the time, the clinic administered a cephalosporin antibiotic (Cefotiam \ Spizef) intravenously for three weeks, which resulted in a largely complete decrease in severe neuralgiform pain ; However, the accompanying chronic fatigue, nocturnal freezing and rapid exhaustion were found to be unsatisfactory. Almost exactly a year later, a recurrence of the original plexus neuritis occurred. Again, intravenous administration of a cephalosporin - this time ceftriaxone - led to a decrease in neuralgiform pain within 10 days, but again not to a decrease in severe exhaustion.Only after repeated therapy cycles lasting several months with the use of different antibiotics (doxycycline,azithromycin) was it finally possible to achieve a satisfactory reduction in severe exhaustion and chronic fatigue.
In the following years I saw patients who developed persistent symptoms such as joint pain, neck pain, headache, drowsiness, exhaustion, etc. after a tick bite with subsequent erythema migrans, but no Lyme disease antibodies. This was the reason to search not only for antibodies against Borrelia burgdorferi (Bb), but also for the pathogen directly in these patients, especially at that time DNA analysis and cultural cultivation of the pathogen from body fluids, but also skin biopsies as a diagnostic method some laboratories were offered. The result of this extended diagnosis is a retrospective case study ( 2 ), which I reported on here 4 years ago.
- The subject of this own retrospective study are 105 patients who came to the practice with clinical suspicion of chronic Lyme borreliosis.
- The effects of longer antibiotic therapy cycles were investigated in patients, some of whom had been given short-term therapy for a few weeks years previously without becoming symptom-free. However, the pathogen could still be detected in skin biopsies and joint punctures. It therefore seemed justified to conclude that the Borrelia infection was still present. Similar results are also reported by Preac-Mursic et al. reported in a study published in 1989 ( 3 ).
Pathogen direct detection was carried out with the help of the determination of
Borrelia DNA by means of polymerase chain reaction (PCR) and / or
by cultivating the pathogen and / or by visualizing the pathogen by means of immunofluorescence microscopy in "focus floating microscopy" technology (from 2007, University Dermatology Clinic Innsbruck) ( 4 ).
The data of 90 patients who were also treated in my practice were evaluated - the remaining 15 cases were only presented for consultation. The aim of treatment in these patients with chronic Lyme disease was the improvement or elimination of the disease state through long-term antibiotic treatment.
Treatment results: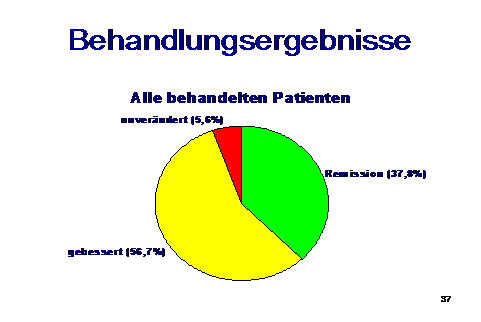
It was shown that even in patients with a history of years or decades, total freedom from symptoms was achievable, and this was at least 37% of the cases.
In 56% of the cases, a satisfactory improvement of the symptoms could be achieved, but despite months of treatment, there were still relapses in these cases, whereby improvement could be achieved again by repeated 3-4 weeks of antibiotics.
In the course of this, there were increasingly longer periods of largely free of symptoms. 5-6% of the treated cases turned out to be therapy-resistant.
The intravenous application of antibiotics (as far as possible from the preparation preparation) proved to be necessary, particularly at the beginning of a respective treatment; If only oral antibiotics were administered, no progress in recovery was generally achievable, particularly in patients with a long history. In many patients, intravenous administration proved to be well tolerated, but oral antibiotics were often less well tolerated. This applies in particular to doxycycline, but also to metronidazole.
An important finding of this retrospective study: Even in patients with decades of disease and a wide range of symptoms, long-term antibiotic treatment, preferably intravenously administered, was able to achieve largely symptom-free intervals of 3-5 years - in many cases longer - as has now been shown in the further observation period. The duration of treatment was a minimum of 6 months and a maximum of 6 years with intermittent antibiotics.
The detection of Bb (DNA analysis and / or positive culture from whole blood) in pretreated patients with disseminated Lyme borreliosis (165 cases) is also provided by J. Oksi et al. reported ( 5 ). In spite of previous antibiosis, this group of authors preferred to apply ceftriaxone for another 4-6 weeks if the pathogen was detected. The success of this repeat treatment was considered good in 9 of 13 relapse patients, but not sufficient in the remaining 4. The authors conclude that treatment for more than three months does not lead to eradication of spirochetes in all cases.
While I mainly practiced antibiotic monotherapy 10-15 years ago, I have used more and more antibiotic combinations over the last 5 years, on the one hand because I noticed that this made faster recovery progress possible, on the other hand from findings from basic research . Important reasons that speak in favor of combination antibiosis: it has been shown that the pathogen can occur in other manifestations in addition to the spiral shape - and not only in the culture, but also in the tissue.
The key words are: cystic form of Bb ( 6 ), granular form of Bb ( 6 ), and cell wall-less form of Bb ( 7 )
The already quoted working group led by Prof. Zelger at the University Dermatology Clinic in Innsbruck was able to show that the spiral form and the cystic form of Bb occur next to each other in skin tissue in Borrelia lymphocytoma . In acrodermatitis patients there were findings in which the granular form of Borrelia burgd. what found.
These largely metabolically inactive forms are not affected by common antibiotics. Only a few substances or groups of substances target these "sleeper forms" and only these can affect these metabolically inactive forms.
Another important finding communicated by Martin Sievers and Priska Keller ( 8 ): “Borrelia are able to grow intracellularly and could be
detected in human endothelial cells, in neuronal cells and in synovial tissue .
However, all of the points mentioned appear to me to be very important when choosing a drug.
A very differentiated description of antibiotics effective against Bb can be found in Walter Berghoff ( 9 ). Antibiotics considered to be effective are listed here under the criteria of
“intracellular effectiveness”,
“CSF penetration”,
“effectiveness on cystic forms” and
“plasma half-life”.
Effective antibiotics for Lyme disease
antibiotic |
|
liquor passage |
effective on cystic forms |
Plasma half-life |
Beta lactams Ceftriaxon |
- - - - |
(+) * + + - |
- - - - |
8 hours 40 min 3 days 1 hour |
Tetracyclines Doxycyclines |
+ |
(+) 14% |
- |
15 hours |
Macrolides Clarithromycin
Telithromycin |
+
+ |
- (2-5%)
- |
-
- |
4 hours |
Gyrase inhibitors Gemifloxacin Tinidazole **
Tygecycline *** |
+ +
+ |
+ (20%) +
+ |
- +
+ |
> 12 hours 10 hours |
|
|
|
|
|
Indication of the duration of action (plasma half-life), the liquor penetration, the intracellular effect and the effect on cystic forms. CSF passage: CSF / serum concentration in%.
* The beta-lactams have poor CSF penetration, but within their wide therapeutic range they reach CSF concentrations that are well above the minimum inhibitory concentration (MIC) (cf. 164).
** Tinidazole and metronidazole act on biofilm (Tinidazole> Metronidazole, Kaur N et al, 2010 (unpublished)).
*** Tygecycline belongs to the class of glycylcycline antibiotics, is highly effective against Borrelia in vitro, can pass through the liquor, acts intracellularly and on cystic forms and has a long plasma half-life, not yet clinically tested in LB (185, 186).
This table appears to me to be extremely helpful when choosing a drug.
It is obvious that a Lyme disease sufferer with a chronic form - for example with acrodermatitis - is confronted not only with the spiral form , but also with the cystic and granular - possibly also without cell wall - form of Borrelia burgdorferi.
For me, this results in the need to use the v. Bb active substances in a therapy regimen also to be used.
The “intracellular effectiveness” criterion of an antibiotic also seems very important to me.
If we look at beta-lactam antibiotics , for example , it becomes clear that they do not meet two important criteria for effectiveness : They are not intracellularly effective and they do not act on the cystic form of the pathogen.
Ultimately, these considerations result in my preferred combination antibiosis; this usually includes hydroxychloroquine 0-0-200 mg orally, followed by doxycycline 200 mg iv-0-100 mg orally or a macrolide antibiotic, followed by metronidazole 500 mg iv-0-500 mg orally.
Mechanisms of action of the individual substance groups:
Metronidazole , from the same group of substances also tinidazole: each DNA strand break ;
Hydroxychloroquine: lysosomotropic substance , indirect effect by changing the intralysosomal acidity - and - with a similar mechanism of action also Artemisia annua (extract from annual mugwort) (lysosomotropic substance).
The substances mentioned are usually combined either with protein synthesis inhibitors from the group of tetracycylines and / or macrolides .
I have also practiced a combination of metronidazole and hydroxychloroquine with beta-lactam antibiotics , which are actually membrane synthesis inhibitors , especially in cases where there was no improvement under tetracyclines and / or macrolides.
In order to make it easier for the patient to assess the tolerance of the individual substances, I do not prescribe the selected preparations at the same time, but in stages.
Usually I start with hydroxychloroquine - alternatively also with Artemisia annua - followed mostly by iv doxycycline, followed by iv metronidazole or oral tinidazole.
Further findings from basic research:
The complex and varied interactions v. Bb with fibers of the connective tissue or with structures of ligaments and tendons - and the literature available on this - were presented by Kurt E. Müller in a review article ( 10 ). According to this, Borrelia grow preferentially on or in biological substrates by breaking down the extracellular matrix ( 11 ), they have metalloproteases with which they can dissolve collagen and thus settle as microcolonies in collagen fibers ( 12 ). With this review, Mr. Müller touched on the phenomenon of "biofilm" or the growth of bacteria in biofilm-like formations.
The preoccupation with the subject of "Lyme disease as a clinical entity" and the subject of "biofilm" as by far the most important way of life of many microorganisms suggest that chronic Lyme disease should be understood as a biofilm infection, especially since the chronic course of the disease can be easily explained.
Microorganisms in the biofilm produce signal molecules, the increase in concentration of which communicates the cell density. This phenomenon is called "quorum sensing".
Quorum sensing is the ability of protozoa to measure the cell density of the population via chemical communication. It allows thecells to become suspendedto activate certain genes only when a certain cell density is exceeded or not reached. As such, the term "quorum "Comes from the time of the Roman Empire and denoted the smallest number of members required for a vote in the Senate.
Andreas Wieser and Sören Schubert ( 13 ) reported on intra- and extracellular biofilms of uropathogenic E. coli bacteria in a paper from August 2011 .
It defines biofilms as a structured community of microorganisms that envelop themselves in a self-made polymeric matrix and that are adherent to a living or artificial surface.
These authors see biofilms as a therapeutic challenge, as the pathogens they contain show strong resistance to a wide variety of environmental influences. This also includes resistance to antimicrobial chemotherapeutic agents and host defense mechanisms during infection.
A biofilm as a special manifestation of Bb was able to be characterized by the research group around Prof. Eva Sapi ( 14 ) using different types of microscopy: Quote / translation from this announcement:
"Among the optical microscopy techniques, dark field microscopy was used to observe the interaction of peripheral spirochetes with the biofilm. DIC microscopy (differential interference contrast microscopy) showed the heterogeneity of the biofilm matrix. Fluorescence microscopy allowed the observation of the sessile internal biofilm population in a GF population Fluorescent protein) -expressing population. A relatively new technique, atomic force microscopy, has been used to scan the topography of biofilms directly. B. burgdorferi's ability to take on a biofilm-like morphology may (in part) reduce the persistent discomfort explain in chronically ill patients: Borrelia burgdorferi- Biofilm presumably offers a retreat for Borrelia in the course of the chronic infection, but also offers an additional avenue of attack for potential treatments of Lyme disease. "
Further findings emerge from the following contribution by the same working group ( 15 ):
Quotation / translation from this statement:
“Our considerations led to the urgent assumption that B. burgdorferiis able to hide in a self-generated protective layer, the so-called biofilm. The main purpose of the biofilm structure is to enable microbes to survive under various environmental stressors, including the presence of attacking immune cells or antibacterial substances. While conventional antibiotic therapy usually proves to be effective against freely occurring bacteria, it is often ineffective when pathogens have formed biofilms, because bacterial colonies in biofilms can be up to 1000 times more resistant to antibiotics. In order to prevent and destroy Borrelia burgdorferi biofilms, we need a better understanding of the molecular mechanisms that take place during the formation of a biofilm.In this project we decided to specific genetic markers that regulate communication in biofilms - so-called “quorum sensing factors” - to be monitored or monitored. …… Our results show that the regulation of the mentioned quorum sensing genes is dynamic and excellent markers for assessing or monitoring the development of Borrelia burgdorferi biofilms ”.
In a paper by Kristen Kerksiek ( 16 ), the following communications on the subject of resistance to antimicrobial agents can be found:
“Microorganisms in biofilms differ from their free-living relatives: They express special genes, have a very heterogeneous metabolism depending on their position within the biofilm and are in close contact, which facilitates gene transfer. The extreme antibiotic resistance of biofilms - they are up to 1000 times more resistant than free-living bacteria of the same strain - caught us off guard, but actually we shouldn't have been surprised: Antibiotics are not tested on biofilms, but on (freely occurring) Plankton bacteria.
The fact that biofilms are so resistant to treatment with antibiotics is probably due, among other things, to the fact that access to the active ingredients is more difficult. More important than the physical barrier of the exopolymers in the biofilm, however, seems to be their structural heterogeneity: they are contained in niches in which the microorganism populations have only low metabolic activity due to the low oxygen and nutrient concentrations, and in such a dormant state bacteria are relatively resistant to antibiotics. Antibiotic treatment may kill the bacteria in the outer layers of the biofilm, where there is higher metabolic activity, but even a few "stubborn" cells can regrow the biofilm as soon as the treatment is stopped; in the outer wall of the biofilm that shields the microorganisms inside,individuals are sacrificed for the community to survive. Such stubborn variants (as opposed to mutations or the spread of a resistance gene) seem to be the most important factor for the antibiotic resistance of the biofilms, because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally. "Because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally." because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally. "Such stubborn variants (as opposed to mutations or the spread of a resistance gene) seem to be the most important factor for the antibiotic resistance of the biofilms, because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally. "Because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally." because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally. "Such stubborn variants (as opposed to mutations or the spread of a resistance gene) seem to be the most important factor for the antibiotic resistance of the biofilms, because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally. "Because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally." because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally. "That means they remain sensitive to antibiotics, even if they were originally. "because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally."That means they remain sensitive to antibiotics, even if they were originally. "because the surviving bacteria still respond to antibiotics. That means they remain sensitive to antibiotics, even if they were originally."
The mechanism of (antibiotic) resistance in biofilms is discussed in a work by PS Stewart and J W. Costerton ( 17 ). It differs from known mechanisms such as genetic changes through plasmids, transposons and mutations, which give individual bacterial cells their own resistance.
In biofilms, antibiotic resistance seems to depend on multicellular strategies.
It describes the "phenomenon of slow penetration" of the antibiotic. The antibiotic fails to get through the outer layer of the biofilm. Some of the bacteria contained in the biofilm can transform into a protected phenotype.
Furthermore, the "phenomenon of the changed microenvironment" is mentioned:
In areas of reduced food supply or the accumulation of metabolic (waste) products, antibiotics may become antagonized.
On the basis of all these findings, in my opinion, it becomes understandable why some infections - including chronic borreliosis - cannot be treated easily and certainly not “quickly”, ie within a few weeks, really successfully.
In the work by A. Wieser and S. Schubert ( 13 ), which has already been cited, “Intracellular biofilms” are also reported:
“Intracellular biofilms are an entity that seems to play a role in urinary tract infections in particular. These are intracellular replicating and persisting pathogens that protect themselves from the harsh environmental conditions and the host's immune system in the urinary tract through this niche. "
The intracellularly observed transformation v. Bb in the “granular form” should correspond to the process of the formation of an intracellular biofilm ( 6 ). The treatment of a pathogen that has settled intracellularly is even more difficult than the treatment of an extracellular or possibly only planktonically occurring pathogen.
The above-mentioned findings about biofilm infections also provide prospects for new therapeutic options:
On the subject of “therapy options” , Kristen Kerksiek ( 16 ) once again says :
“Working with biofilms in the laboratory is difficult, but after realizing how important they are in connection with infectious diseases, research made a lot of progress. A big step was the realization that the formation of biofilms is a genetically programmed development process; This opened up the possibility of specifically developing new chemotherapeutic agents.
There are various strategies for finding effective treatment methods for biofilm infections: For example, you can prevent the bacterial cells from sticking together, or you can reduce the production of polysaccharides and disrupt cell-cell communication. In particular, the discovery of quorum sensing communication gave rise to great hopes that newly developed active ingredients could prevent the formation of biofilms or dissolve existing biofilms. “
This also corresponds to the quoted approach of Eva Sapie and her working group ( 15 ).
Further aspects on the subject of "therapy options " quoted from Kristen Kerksiek ( 16 ):
“Biofilms from Staphylococcus aureus communicate with auto-inducing peptides (AIPs) in quorum sensing. Horswill and colleagues ( 18 ) synthesized such peptides and added them to existing biofilms of S. aureus . These then dissolved very quickly and the released bacteria responded again to antibiotics. "
“The slimy extracellular matrix of the biofilms protects the microorganisms from an abundance of enemies, such as the immune system, microbe-inhibiting agents and most bacteriophages (viruses that infect bacteria). Lu and Collins ( 19 ) constructed genetically modified bacteriophages that secrete mucus-degrading enzymes and thus penetrate the interior of the biofilm. There they further destroy the biofilm by producing excessive amounts of the enzymes and then killing the bacteria. "
Raphael B. Stricker and Lorraine Johnson ( 20 ) in January 2011 gave an excellent overview of the findings on the subject of "Borreliosis as a persistent infection" and current research directions - diagnosis and therapy of Lyme disease - which were also addressed in this presentation Title "Lyme disease: the next decade" published.
literature
1) Burrascano, J., Guidelines for Lyme and other tick borne Illnesses, Sixteenth Edition, 2008 Diagnostic hints and treatment, http://www.ilads.org/lyme_disease/B_guidelines_12_17_08.pdf
2) B.-D. Huismans, W. Klemann, Long-term treatment with anti-infectives for persistent Borreliosis with Borrelia DNA detection by PCR , Grin Verlag, October 20, 2008
3) Preac-Mursic, V. et al., Grosshadern Neurological Clinic, Munich, Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis, Infection. 1989 Nov-Dec; 17 (6): 355-9. http://www.ncbi.nlm.nih.gov/pubmed/2613324
4) Eisendle K ., Grabner T ., Zelger B . Fo cus floating microscopy: "gold standard" for cutaneous borreliosis? Am J Clin Pathol. 2007 Feb; 127 (2): 213-22.
5) Oksi J., Marjamäki M., Nikoskelainen J., Viljanen MK. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann Med. 1999 Jun; 31 (3): 225-32.
6) Alan B. Mac Donald, MD, July 7, 2008 University of New Haven Lyme Disease Symposium New Haven, Conn. http://www.molecularalzheimer.org/files/Biofilm_New_Haven_final_lecture.pdf
7) Lida H. Mattman, 3rd Edition, Cell Wall deficent forms Stealth Pathogens, CRC Press LLC, 2001
8) Martin Sievers and Priska Keller (2008), determination of suitable antibiotics against the pathogens of Lyme borreliosis in the cell culture model http://bsg-sw.gmxhome.de/Sievers%20AB%20im%20Zellkulturmodell.pdf
9) Berghoff, W., Antibiotic treatment of Lyme borreliosis (LB), Umwelt -medizin-Gesellschaft (22) 2/2009, ISSN 1437-2606, pp. 125-131 http://www.praxis-berghoff.de / documents / Lyme_Borreliosis_in_Oeberblick.pdf
10) Müller, KE (2009), Disease of the elastic and collagen fibers in Lyme borreliosis, Umwelt · Medizin · Gesellschaft - 2/2009
11) Coleman, JL. et al. (1999), Plasmin-coated borrelia Burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect Immun (8): 3929-3936
12) Zambrano MC , et al. (2004) Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices, Infect Immun. ; 72 (6): 3138-46.
13) Andreas Wieser and Sören Schubert (2011), Intra- and extracellular biofilms of uropathogenic E. coli , Chemother J 2011; 20: 181–5.
14) Luecke DF et al., (2009) Novel Fugitive Strategy for Borrelia burgdorferi: Biofilm http://www.lymeneteurope.org/forum/viewtopic.php?f=5&t=2776
15) Bien-Aim H. et al. (2011), Expression Profile of Quorum Sensing Biomarkers during Biofilm Development in Borrelia burgdorferi, http://www.lymeneteurope.org/forum/viewtopic.php?f=5&t=3468
16) Kerksiek K. (2008), Life in Slime: Biofilms Rule the World. http://www.infection-research.de/de/perspectives/detail/pressrelease/a_life_in_slime_biofilms_rule_the_world-2/
17) Philip S Stewart, J William Costerton, Antibiotic resistance of bacteria in biofilms, Center for Biofilm Engineering and Department of Chemical Engineering, Montana State University, Bozeman, USA; Lancet 2001; 358: 135-38
18) Blaise R. Boles, Alexander R. Horswill, agr -Mediated Dispersal of Staphylococcus aureus Biofilms, PLoS Pathog. 2008 April; 4 (4)
19) Timothy K. Lu and James J. Collins , Dispersing biofilms with engineered enzymatic bacteriophage, PNAS July 3, 2007 vol. 104 no.27 11197-11202
20) Raphael B. Stricker, Lorraine Johnson, International Lyme and Associated Diseases Society, Bethesda, MD, USA; Lyme disease: the next decade. Infection and Drug resistance, 6th January 2011
Terms of use and disclaimer
According to the judgment of May 12, 1998 - 312 O 85/98 - "Liability for Links", Regional Court (LG) Hamburg, the following declaration is issued: As a precaution, the author expressly distances himself from all contents of the linked external Internet pages and does not block these contents Own. This declaration applies to all attached links.
Under no circumstances can the contribution replace a visit to the doctor. The article was created with the greatest care. However, the author cannot accept responsibility for the accuracy or correctness of the information provided. Under no circumstances is the author of the contribution to be held liable for any loss and / or damage that the user could suffer as a result of relying on information obtained in the course of using the contribution.
The author is not financially dependent. This article was created without financial support.